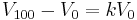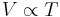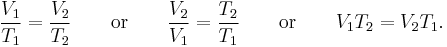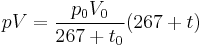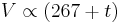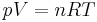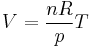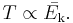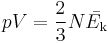Charles's law
Charles' law (also known as the law of volumes) is an experimental gas law which describes how gases tend to expand when heated. It was first published by French natural philosopher Joseph Louis Gay-Lussac in 1802,[1] although he credited the discovery to unpublished work from the 1780s by Jacques Charles. The law was independently discovered by British natural philosopher John Dalton by 1801, although Dalton's description was less thorough than Gay-Lussac's.[2] The basic principles had already been described a century earlier by Guillaume Amontons.
Taylor Buchanan was the first to demonstrate that the law applied generally to all gases, and also to the vapours of volatile liquids if the temperature was more than a few degrees above the boiling point. His statement of the law can be expressed mathematically as:
where V100 is the volume occupied by a given sample of gas at 100 °C; V0 is the volume occupied by the same sample of gas at 0 °C; and k is a constant which is the same for all gases at constant pressure. Gay-Lussac's value for k was 1⁄2.6666, remarkably close to the present-day value of 1⁄2.7315.
A modern statement of Charles' law is:
At constant pressure, the volume of a given mass of an ideal gas increases or decreases by the same factor as its temperature on the absolute temperature scale (i.e. the gas expands as the temperature increases).[3]
which can be written as:
where V is the volume of the gas; and T is the absolute temperature. The law can also be usefully expressed as follows:
The equation shows that, as absolute temperature increases, the volume of the gas also increases in proportion.
Contents |
Charles's Law
When a given mass of a gas is heated at constant pressure, the volume V of given mass of a gas is directly proportional to its absolute temperature.
Relation to the ideal gas law
French physicist Émile Clapeyron combined Charles's law with Boyle's law in 1834 to produce a single statement which would become known as the ideal gas law.[4] Claypeyron's original statement was:
where t is the Celsius temperature; and p0, V0 and t0 are the pressure, volume and temperature of a sample of gas under some standard state. The figure of 267 came directly from Gay-Lussac's work: the modern figure would be 273.15. For any given sample of gas, p0V0⁄267+t0 is a constant (Clapeyron denoted this constant R, and it is closely related to the modern gas constant); if the pressure is also constant, the equation simplifies to
as required.
The modern statement of the ideal gas law is:
where n is the amount of substance of the gas sample; and R is the gas constant. The amount of substance is constant for any given gas sample so, at constant pressure, the equation rearranges to:
where nR⁄p is the constant of proportionality.
An ideal gas is defined as a gas which obeys the ideal gas law, so Charles's law is only expected to be followed exactly by ideal gases. Nevertheless, it is a good approximation to the behaviour of real gases at relatively high temperatures and relatively low pressures.
Relation to absolute zero
Charles' law appears to imply that the volume of a gas will descend to zero at a certain temperature (−266.66 °C according to Gay-Lussac's figures) or -273°C. Gay-Lussac was clear in his description that the law was not applicable at low temperatures:
but I may mention that this last conclusion cannot be true except so long as the compressed vapors remain entirely in the elastic state; and this requires that their temperature shall be sufficiently elevated to enable them to resist the pressure which tends to make them assume the liquid state.[1]
Gay-Lussac had no experience of liquid air (first prepared in 1877), although he appears to believe (as did Dalton) that the "permanent gases" such as air and hydrogen could be liquified. Gay-Lussac had also worked with the vapours of volatile liquids in demonstrating Charles's law, and was aware that the law does not apply just above the boiling point of the liquid:
I may however remark that when the temperature of the ether is only a little above its boiling point, its condensation is a little more rapid than that of atmospheric air. This fact is related to a phenomenon which is exhibited by a great many bodies when passing from the liquid to the solid state, but which is no longer sensible at temperatures a few degrees above that at which the transition occurs.[1]
The first mention of a temperature at which the volume of a gas might descend to zero was by William Thomson (later known as Lord Kelvin) in 1848:[5]
This is what we might anticipate, when we reflect that infinite cold must correspond to a finite number of degrees of the air-thermometer below zero; since if we push the strict principle of graduation, stated above, sufficiently far, we should arrive at a point corresponding to the volume of air being reduced to nothing, which would be marked as −273° of the scale (−100/.366, if .366 be the coefficient of expansion); and therefore −273° of the air-thermometer is a point which cannot be reached at any finite temperature, however low.
However, the "absolute zero" on the Kelvin temperature scale was originally defined in terms of the second law of thermodynamics, which Thomson himself described in 1852.[6] Thomson did not assume that this was equal to the "zero-volume point" of Charles's law, merely that Charles's law provided the minimum temperature which could be attained. The two can be shown to be equivalent by Ludwig Boltzmann's statistical view of entropy (1870).
Relation to kinetic theory
The kinetic theory of gases relates the macroscopic properties of gases, such as pressure and volume, to the microscopic properties of the molecules which make up the gas, particularly the mass and speed of the molecules. In order to derive Charles's law from kinetic theory, it is necessary to have a microscopic definition of temperature: this can be conveniently taken as the temperature being proportional to the average kinetic energy of the gas molecules, Ek:
Under this definition, the demonstration of Charles's law is almost trivial. The kinetic theory equivalent of the ideal gas law relates pV to the average kinetic energy:
where N is the number of molecules in the gas sample. If the pressure is constant, the volume is directly proportional to the average kinetic energy (and hence to the temperature) for any given gas sample. ... absolute zero is in attainable in gases because most of the gases turn to liquids i.e. they leave the state of gas thus the law is not valid . This is only a theoretical limitation and thus works in practice.
Applications of Charles's Law
- Bursting of hydrogen balloon
- Making of chappathi
See also
References
- ^ a b c Gay-Lussac, J. L. (L'An X – 1802), "Recherches sur la dilatation des gaz et des vapeurs", Annales de chimie XLIII: 137. English translation (extract).
- ^ http://www.chemistryexplained.com/Fe-Ge/Gay-Lussac-Joseph-Louis.html
- ^ Fullick, P. (1994), Physics, Heinemann, pp. 141–42, ISBN 0435570781.
- ^ Clapeyron, E. (1834), "Mémoire sur la puissance motrice de la chaleur", Journal de l'École Polytechnique XIV: 153–90. Facsimile at the Bibliothèque nationale de France (pp. 153–90).
- ^ Thomson, William (1848), "On an Absolute Thermometric Scale founded on Carnot's Theory of the Motive Power of Heat, and calculated from Regnault's Observations", Philosophical Magazine: 100–6, http://zapatopi.net/kelvin/papers/on_an_absolute_thermometric_scale.html.
- ^ Thomson, William (1852), "On the Dynamical Theory of Heat, with numerical results deduced from Mr Joule's equivalent of a Thermal Unit, and M. Regnault's Observations on Steam", Philosophical Magazine 4. Extract.
Further reading
- Krönig, A. (1856), "Grundzüge einer Theorie der Gase", Annalen der Physik 99: 315–22, Bibcode 1856AnP...175..315K, doi:10.1002/andp.18561751008. Facsimile at the Bibliothèque nationale de France (pp. 315–22).
- Clausius, R. (1857), "Ueber die Art der Bewegung, welche wir Wärme nennen", Annalen der Physik und Chemie 176: 353–79, Bibcode 1857AnP...176..353C, doi:10.1002/andp.18571760302. Facsimile at the Bibliothèque nationale de France (pp. 353–79).</ref>
- Joseph Louis Gay-Lussac – Liste de ses communications, http://www.polytechnique.fr/bcx/associations/gaylussac/pages/CommunicationsGL.html . (French)
External links
- Charles's law simulation from Davidson College, Davidson, North Carolina
- Charles's law simulation from TutorVista.com
- Charles's law demonstration by Prof. Robert Burk, Carleton University, Ottawa, Canada
- Charles's law animation from the Leonardo Project (GTEP/CCHS, UK)